How does the COVID-19 prevention drug Evusheld work and who should receive it?
Patrick Jackson, University of Virginia
The U.S. Food and Drug Administration granted emergency use authorization to AstraZeneca’s COVID-19 antibody drug Evusheld on Dec. 8, 2021. Infectious disease physician Patrick Jackson of the University of Virginia explains how it works, who’s eligible and why some patients are having difficulties accessing it.
1. What is Evusheld, and how does it work?
Evusheld is the first FDA-authorized drug to prevent COVID-19 in high-risk people who aren’t adequately protected by vaccination alone. Data from a preliminary study that has not yet been peer reviewed showed Evusheld reduced the risk of symptomatic COVID-19 by 77% in unvaccinated high-risk adults.
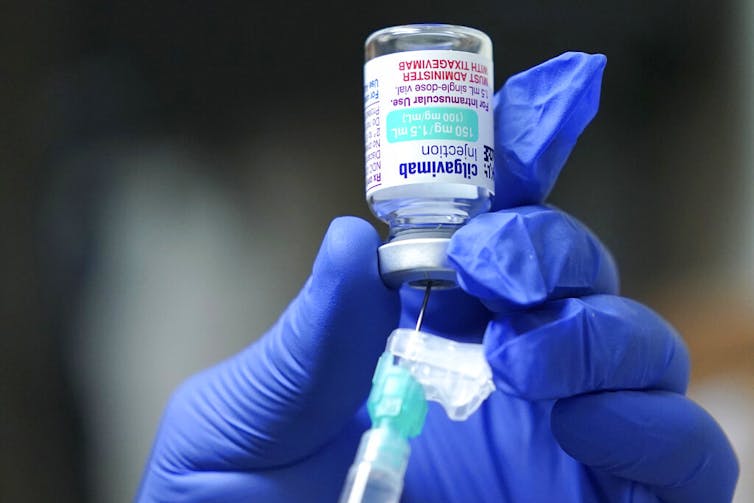
When the immune system is exposed to a foreign protein – for example, by infection or vaccination – it produces antibodies in response to the potential threat. Evusheld is a combination of two antibodies, tixagevimab and cilgavimab, that bind to the spike protein of the virus that causes COVID-19 and prevent it from entering and infecting cells. Evusheld is a monoclonal antibody drug, meaning that it is made of mass-produced identical antibodies that originally came from a single type of white blood cell. Evusheld functions differently from antiviral drugs like molnupiravir, which work by stopping the virus from replicating within cells.
Tixagevimab and cilgavimab are versions of natural human antibodies that have been modified to last much longer in the body than they normally would. This allows Evusheld to provide COVID-19 protection for several months following a single dose. It is expected that Evusheld will need to be given about every six months to keep antibody levels high enough to be effective against the virus. Patients may need to keep getting Evusheld doses as long as there is a significant risk of COVID-19.
Evusheld is not intended to treat COVID-19, but to help prevent vulnerable patients from getting sick in the first place.
2. Who should be receiving Evusheld?
Evusheld can be used by people ages 12 and up who fall into two specific groups unable to receive the full benefit of COVID-19 vaccination.
The first group is people who are moderately to severely immunocompromised because of a medical condition or treatment. While most in this group get some protection from the COVID-19 vaccines, the immune systems of some may not be able to make enough protective antibodies on their own. This includes people receiving treatment for certain cancers, solid organ or stem cell transplant recipients and people with certain immune system disorders. People who take immunosuppressive medications, such as high-dose steroids and common autoimmune disease treatments, may also be eligible.
Evusheld is also authorized for the small number of people who had a severe reaction to the COVID-19 vaccines and can’t receive the full recommended dose regimen. It is important to note that this does not apply to common mild reactions, such as pain at the injection site or mild fever. Most people who have rare allergic reactions to the COVID-19 vaccines can still safely receive additional doses, and should discuss their options with their doctor.
3. When are you supposed to take Evusheld?
Evusheld is used to prevent COVID-19 before a person has been exposed to the virus. Currently it isn’t approved to treat someone who is already sick with COVID-19 or to prevent an infection after recent exposure.
There are several COVID-19 treatments available for high-risk people who do become infected. Unpublished data that have not yet been peer reviewed suggest that Evusheld may have a role in COVID-19 treatment in addition to prevention, but using the drug in this way has not yet been authorized by the FDA.
Evusheld is given at least two weeks after a patient’s last vaccine dose. This is to ensure the vaccine has had enough time to establish its full protective effects. This recommendation may change as researchers learn more about how vaccines and monoclonal antibodies like Evusheld work together.
Generally, immunocompromised people who can get vaccinated and boosted for COVID-19 should do so in addition to taking Evusheld. While they may not be as strongly protected as others, vaccination is still likely to provide some benefit.
4. How effective is Evusheld against variants?
One significant shortcoming of monoclonal antibody drugs like Evusheld is that they are not all equally effective against different variants of the virus that causes COVID-19.
Evusheld entered clinical trials before the omicron variant dominated infections around the world. Lab studies have given conflicting results on how effective Evusheld might be against the omicron subvariants currently circulating in the U.S. It also isn’t clear how well those lab studies predict real-world protection against COVID-19.
In response to this concern, the FDA recently doubled the authorized dose of Evusheld. The idea is that if the Evusheld antibodies are less effective against one of the omicron subvariants, more antibodies might still offer protection. Future variants may make Evusheld more or less effective.
5. Are there any other preventive treatments?
Other than the vaccines, Evusheld is currently the only drug approved or authorized in the U.S. for the prevention of COVID-19.
Until recently, two other monoclonal antibody drugs, casirivimab-imdevimab and bamlanivimab-etesevimab, were used to prevent disease in people who were recently exposed to COVID-19. Unfortunately, these drugs are not effective against the omicron variant that is now the source of almost all U.S. COVID-19 cases.

Researchers are looking into whether another monoclonal antibody, sotrovimab, which is currently being used as a treatment for COVID-19 in certain U.S. regions that have not yet been overtaken by the BA.2 omicron subvariant, could also be used to bolster immunity in immunocompromised people.
There is no evidence that drugs like hydroxychloroquine or ivermectin are useful for preventing COVID-19.
6. Why is it so difficult to access Evusheld?
The U.S. government has purchased hundreds of thousands of doses of Evusheld and is distributing these through state and territorial health departments. But that’s far fewer doses than the 7 million or more immunocompromised people, or roughly 2.7% of American adults, who might benefit from this drug. While AstraZeneca has said there are more doses, it is unclear whether the U.S. plans to purchase more.
While some hospitals have had overwhelming demand, others have unused doses. Some hospitals have had to implement allocation systems to ensure that patients at highest risk are prioritized, and those policies are not standardized. The recent FDA decision to increase the standard Evusheld dose also means that supply won’t be able to stretch as far.
Unfortunately, because Congress has failed to fund ongoing COVID-19 programs, this might further decrease the supply of Evusheld available to patients.
7. How do I know if I need Evusheld, and how can I get it?
If you think you might benefit from Evusheld, talk to your doctor about whether you qualify. The doctor can write you a prescription.
Evusheld is administered as two injections during one session, and patients are observed for one hour to monitor for rare allergic reactions. Because of limited supply and these special monitoring requirements, Evusheld is given only at certain locations. Many state health departments have websites that let you look up nearby medical centers that have Evusheld. The federal government also has a treatment locator for Evusheld and other COVID-19 drugs, though this may not be completely up to date.
[Get fascinating science, health and technology news. Sign up for The Conversation’s weekly science newsletter.]![]()
Patrick Jackson, Assistant Professor of Infectious Diseases, University of Virginia
This article is republished from The Conversation under a Creative Commons license. Read the original article.