Despite its disastrous effects, COVID-19 offers some gifts to medicine
Dario Ghersi, University of Nebraska Omaha
For all the misery that the pandemic has wrought, it has also opened up a vast storehouse of knowledge about medical issues beyond COVID-19. While it’s still too early to draw conclusions, evidence is emerging of links between autoimmune disorders and the virus that causes COVID-19.
As a bioinformatics researcher with medical training and expertise in immune system modeling, I find this development especially exciting.
The immune system is the most powerful weapon against infection. But on rare occasions, something devastating happens: The immune system turns against its own body – a condition that researchers call autoimmunity. This can result in any of a wide range of autoimmune disorders. They include rheumatoid arthritis, multiple sclerosis and lupus, an inflammatory disease in which the immune system attacks multiple tissues.
Researchers are still trying to solve the mystery of what causes these diseases, in hopes of developing therapies to treat them. COVID-19 may accelerate that process by giving researchers new insight into old findings about the immune system.
From autoimmunity to COVID-19
Molecules called interferons are a significant component of the body’s defense against viruses. These proteins are especially important in the early stages of an infection, frequently getting ahead of it before symptoms appear. Immune cells produce interferons, which then do what their name implies – literally run interference when a virus begins to multiply. At least that’s what they’re supposed to do.
But reports from early in the pandemic showed that in some patients with severe COVID-19, one interferon, known as Type I, showed a weak response to the virus. Some patients actually developed antibodies specifically targeting Type I interferons – essentially knocking out one of the body’s first lines of defense against the disease.
Researchers had discovered similar occurrences decades before. After introducing interferons to treat a patient with cancer in 1980, doctors found that some of the patient’s antibodies were effectively neutralizing those interferons. And in 1982, researchers reported that antibodies had disarmed the interferons in a patient with lupus. Interferon-fighting antibodies could explain some severe COVID-19 cases.
Another explanation is that, instead of becoming weaker in the face of COVID-19, interferons mount a stronger-than-usual defense, inducing organ damage while fighting the virus. Researchers are investigating the possibility that – for patients with the worst cases – Type I interferon is COVID-19’s all-or-nothing double-edged sword: either rendered inactive before it can fight the infection, or somehow becoming hyperactive and potentially detrimental to the body in later stages.
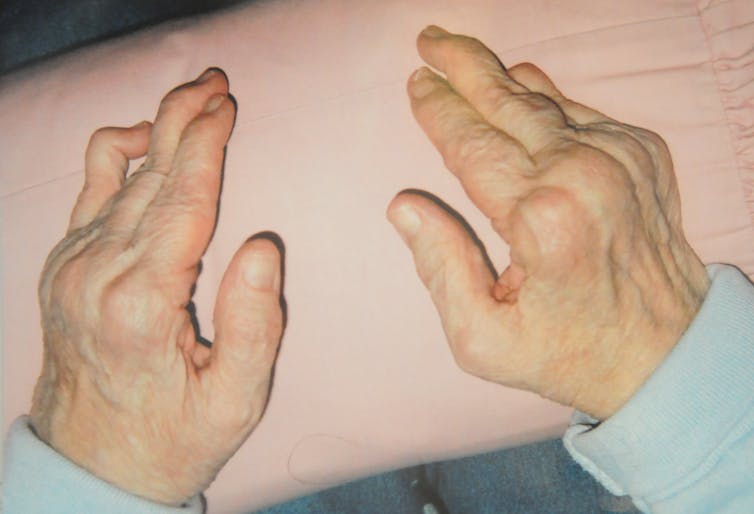
A window into autoimmunity
Throughout the pandemic, doctors have also noticed that patients with severe COVID-19 infections have symptoms that are similar to autoimmune disorder symptoms, such as blood vessel inflammation, rashes and organ damage. Following COVID-19 infection, some patients have even developed full-blown autoimmune disorders, such as Type 1 diabetes, lupus and psoriatic arthritis, a disease marked by skin rashes along with stiff, swollen and painful joints.
Some immunologists suspect that the SARS-CoV-2 virus may be triggering the body to attack itself with autoantibodies – or antibodies that target the body’s own tissues. This could explain why some people who had COVID-19 later developed autoimmune disorders.
It’s not the first time researchers have suggested a possible connection between viruses and autoimmune disorders. For example, a 2019 study of patients with Type 1 diabetes found that those patients also carried several gastrointestinal viruses.
Immunologists are now looking more closely at other viruses and their possible involvement in autoimmune disorders. One example is the Epstein-Barr virus, or EBV, which is responsible for infectious mononucleosis. This virus causes swollen lymph nodes, fever, sore throat and persistent tiredness. Studies in the past two years suggest that the Epstein-Barr virus might also play a role in causing multiple sclerosis and lupus.
So how could COVID-19 cause autoimmunity? One theory is that the virus makes immune cells hyperactive. For example, a computational analysis identified a section of the virus that looks like part of a dangerous type of strep bacteria. This could cause an extreme reaction as the immune system gears up to fight a particularly powerful enemy.
Pieces of the SARS-CoV-2 virus can also mimic parts of human proteins, such as coagulation factors, which regulate bleeding. In some people, the immune system responds by going after those lookalikes. The resulting autoimmune reactions could be causing symptoms like the blood clots and multiorgan damage occurring in patients with COVID-19.
The long view
The condition commonly known as “long COVID-19” is characterized by persistent tiredness, difficulty in concentrating, shortness of breath and a plethora of other symptoms. Interestingly, the symptoms of long COVID bear a strong resemblance to myalgic encephalomyelitis, or ME. More commonly known as chronic fatigue syndrome, ME is a condition characterized by extreme tiredness, pain, sleep problems and a lack of concentration. Long COVID includes some of the same symptoms.
A 2021 study suggested that in both illnesses, the symptoms may be the work of autoantibodies, or antibodies that attack the immune system. Another study found autoantibodies in patients with long COVID who were experiencing cognitive symptoms, like having trouble concentrating.
[Over 140,000 readers rely on The Conversation’s newsletters to understand the world. Sign up today.]
There is now more work under way to further decipher the relationship between autoimmune disorders and viral infections like COVID-19. And doctors are now looking at new therapies for controlling an overreactive immune system.
In the past two years, the pandemic has given medical science an extraordinary amount of knowledge, with more to come.![]()
Dario Ghersi, Associate Professor of Biomedical Informatics, University of Nebraska Omaha
This article is republished from The Conversation under a Creative Commons license. Read the original article.